Pesticide Resistance Management

Pesticide resistance in this age of vector-borne disease can hamper the ability of public health officials to successfully control threats. Potential resistance may also lead to the reemergence of several diseases that would have been otherwise contained through control measures Brogdon 1998[1]. Current resistance in select mosquito populations may be the result of historical insecticide use in the agricultural and pest control industries Rodriguez 2005[2].
Although examples of pesticide resistance in many insect species have been well documented, the scope of the issue and its genuine impact on public health control activities for mosquitoes is not well known. The uncertainties surrounding insecticide resistance have supported CMMCP efforts to monitor for detection of early resistance. In the case of observed resistance, adulticide protocols could be modified to ensure continued efficacy. CMMCP has conducted resistance management since 2006. No detectable resistance to any of our adulticide products have been noted during these tests.
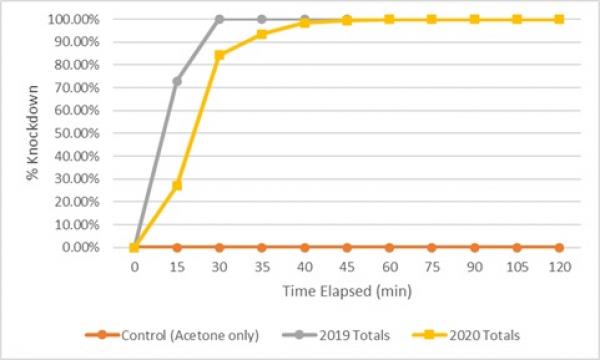
The species composition of the field collected mosquitoes used during the 2020 bottle bioassays were overwhelmingly Coquillettidia perturbans. Although the CDC has not published a standardized time estimate for 100% knockdown of Cq. perturbans, they have set a standard etofenprox concentration per bottle of 12.5µg regardless of species. The published mortality times for six species (Ae. aegypti, Ae. albopictus, Cx. molestus, Cx. pipiens, Cx. tarsalis, and Cx. quinquefasciatus) range from 15-105 minutes for etofenprox. All of these knockdown estimates from the CDC are determined using lab reared mosquitoes. This is somewhat problematic as CMMCP utilizes mosquitoes collected from surveillance traps and these specimens from the field are typically not all of the same species, nor are they of the same age or metabolic characteristics. These variables can create different resistance outcomes during the bottle bioassays. To help address the issues present with using field collected mosquitoes, CMMCP staff is planning on collecting egg rafts from local larval habitats in 2021, rearing the larvae, and using the adults in bottle bioassays. If this method is successful it will eliminate the potential age discrepancies of the specimens, feeding stage, and species composition.